
Reifen-Lexikon von A - Z
Abrollumfang:
Der Abrollumfang bezeichnet die mit einem Reifen zurückgelegte Wegstrecke bei einer kompletten Drehung um 360°. Der Umfang ist abhängig vom Reifendurchmesser.
Alterung:
Reifen altern aufgrund physikalischer und chemischer Prozesse beispielsweise durch Witterungseinflüsse wie UV-Licht, Feuchtigkeit sowie extrem hohen oder niedrigen Temperaturen. Dadurch verändern sich Elastizität und Haftfähigkeit des Reifens. Das gilt auch für nicht oder wenig benutzte Reifen. Um diesem Prozess entgegenzuwirken, werden dem Material Substanzen zugegeben, die den Alterungsprozess stark verlangsamen. Damit ist gewährleistet, dass auch ein, mehrere Jahre (maximal 5 Jahre) sachgemäß gelagerter Reifen der Spezifikation eines Neureifens entspricht und in seiner Verwendungstauglichkeit nicht beeinträchtigt ist. Es wird empfohlen, Reifen nach zehn Jahren durch Neue zu ersetzen.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Aquaplaning:
Beim Aquaplaning schiebt sich wegen erhöhten Wasserstandes auf der Straße ein Wasserkeil zwischen die Reifenaufstandsfläche und dem Fahrbahnuntergrund. Dieses Wasser kann nicht schnell genug abfließen. In Extremfällen verlieren hierbei einzelne oder alle Reifen vollständig den Fahrbahnkontakt. D.h., sie können keine Lenk- und Bremskräfte mehr übertragen. Das Fahrzeug ist nicht mehr steuerbar. Auch Sicherheitsassistenzsysteme wie ABS oder ESP können hier nicht mehr regulierend eingreifen. Aquaplaning wird von den folgenden Faktoren beeinflusst: Fahrgeschwindigkeit, Profiltiefe, Reifenbreite, Reifendruck und Wassertiefe.
Tipps für den Umgang und der Vorbeugung von Aquaplaning hat der ADAC hier zusammengestellt:
https://www.adac.de/infotestrat/reifen/rund_um_den_reifen/aquaplaning/default.aspx
Auswuchten:
Aufgrund von Ungleichgewichten am Rad-Reifen-System durch unterschiedliche Massenverteilung muss ein montierter Reifen ausgewuchtet werden, um einen optimalen Rundlauf zu gewährleisten. Hierzu werden kleine Gewichte an der Felge angebracht. Schlecht ausgewuchtete oder nicht ausgewuchtete Räder beanspruchen Reifen, Radlager und Radaufhängung übermäßig.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Bremsplatten:
Als Bremsplatten wird die Stelle eines Reifens bezeichnet, welcher durch ein blockierendes Rad sehr stark abgenutzt ist. Meist ist die Beschädigung so stark, dass der Reifen gewechselt werden sollte. Moderne Kraftfahrzeuge verfügen über ein Anti-Blockier-System (ABS), welches zuverlässig das Blockieren der Räder bei einer Vollbremsung verhindert.
C-Reifen
Die Abkürzung „C“steht für Commercial und bezeichnet Reifen mit verstärkter Karkasse. Durch die damit verbundene höhere Tragfähigkeit eignen sich diese Reifen meist für Transporter und Vans. C-Decken finden Sie in unserem Reifen-Onlineshop.
Cordfäden
Die Karkasse besteht aus feinen Cordfäden, die früher aus Baumwolle bestanden, heute aber hauptsächlich aus Kunstfaser (im wesentlichen Rayon) bestehen. Die Cordfäden werden gummiert und tragen auf der, der Innenseite des Reifens zugewandten Seite zur Abdichtung eine spezielle Gummischicht. Vom Verlauf der Cordfäden von Wulst zu Wulst hängt die Reifenbauart ab: Sind die Fäden schräg zur Fahrtrichtung angeordnet, handelt es sich um Diagonalreifen, wenn sie quer angeordnet sind, also im rechten Winkel zur Laufrichtung, spricht man von Radialreifen.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
DOT Nummer
Das Datum der Reifenherstellung muss gemäß ECE-Regelung Nr. 30 Punkt 3.1.9 sowie § 36 StVZO dauerhaft auf einer Flanke des Reifens angebracht sein. DOT ist die Abkürzung für Department of Transportation, dem amerikanischen Verkehrsministerium, welches diese Nummer für alle ab den 80'er Jahren produzierten Reifen einführte. Alle namhaften Hersteller übernahmen diese Nummer. Anhand der vierstelligen DOT-Nummer können Sie das Produktionsdatum des Reifens ablesen. Die ersten beiden Ziffern geben die Kalenderwoche, die letzten beiden das Jahr an. Beispiel: 0815, 8. Woche 2015.
Laut dem Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV) gelten unter der Voraussetzung einer sach- und fachgerechten Lagerung Reifen bis zu einem Alter von 3 Jahren als fabrikneu und bis zu maximal 5 Jahren als neu. Bedenken Sie beim Reifenkauf für Ihren Oldtimer, dass die meisten Reifengrößen nur unregelmäßig in geringen Stückzahlen produziert werden und somit nicht immer verfügbar sind.
Diagonalreifen
Die Anordnung der Cordfäden im Reifen bestimmt seine Aufbauart. Bei Diagonalreifen sind die Cordfäden schräg im 45°-Winkel zur Fahrtrichtung angeordnet. Bei Radialreifen sind diese hingegen quer zur Laufrichtung angeordnet. Zeitlich lassen sich Diagonalreifen ungefähr so einordnen:
1928-1940 Diagonalreifen mit Wulstkern
1940-1968 Diagonal(ballon)reifen
Ihren Diagonalreifen finden Sie in unserem Reifen-Shop.
Ein Bericht aus der Zeit zu den Unterschieden von Diagonalreifen und den "neuen" Radialreifen - vom 14.11.1969 "Gürtelreifen‚Wunderreifen?: Mehr „Spitze“: http://www.zeit.de/1969/46/mehr-spitze/komplettansicht.
Drehmoment
Radbolzen, Radschrauben und Radmuttern sollten immer gleichmäßig, über Kreuz angezogen werden. Beachten Sie das richtige Drehmoment und benutzen Sie hierbei einen hochwertigen Drehmomentschlüssel und keinen Druckluftschrauber. Das richtige Drehmoment entnehmen Sie der Betriebsanleitung Ihres Wagens oder der ABE der Felgen. Montierte Reifen sollten immer nach 50 km nachgezogen werden.
Dynamische Unwucht
Eine dynamische Unwucht bezeichnet eine inhomogene Massenverteilung am Rad, welche durch Dynamik (Bewegung) auftritt. Eine dynamische Unwucht lässt sich mit einer Auswuchtmaschine feststellen, da der Rundlauf dabei dynamisch geprüft wird. Der Ausgleich einer Unwucht erfolgt durch Anbringen von Gewichten an der Felge.
Einpresstiefe
Mit der Einpresstiefe (auch abgekürzt ET) – bei manchen Radherstellern auch „IS“ oder „Offset“ – eines Kraftfahrzeugrades wird der Abstand zwischen Felgenmitte (gemessen zwischen den Felgenhörnern) und der inneren Auflagefläche des Radflansches bezeichnet. Das Maß wird in Millimetern angegeben und kann sowohl positive als auch negative Werte annehmen. Andere Maße von Rädern und Felgen werden in Zoll angegeben. Die entsprechende Auflagefläche des Fahrwerkes, der Nabenflansch, kann auch die Bremstrommel oder die Bremsscheibe aufnehmen.
Quelle: https://de.wikipedia.org/wiki/Einpresstiefe
ECE-Kennzeichnung
ECE bedeutet „Economic Commission for Europe“ (Wirtschaftskommission für Europa der Vereinten Nationen). Die Ziffer hinter dem „E“ gibt Auskunft über das Land in dem die Prüfung bzw. die Genehmigung durchgeführt wurde. Deutschland hat die Kennung „E1“.
ECE Regelung Nr. 30
Einheitliche Bedingungen für die Genehmigung der Luftreifen für Kraftfahrzeuge und ihre Anhänger
Diese internationale Richtlinie, die durch die "E-Kennzeichnung" oder auch "E-Nummer" auf dem Reifen belegt wird, besagt, dass der Reifen die Prüfkriterien nach ECE 30 bestanden hat. Ab dem Herstellungsdatum 01.10.1998 produzierte Reifen dürfen nicht mehr ohne "E-Nummer" gehandelt und im öffentlichen Straßenverkehr gefahren werden. Von dieser Regelung ausgenommen sind Reifen, die bestimmt sind für die Ausrüstung historischer Fahrzeuge (Oldtimer). Ein Fahrzeug musss mindestens 30 Jahre alt sein, um als Oldtimer zu gelten (H-Kennzeichen).
Quelle: http://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=CELEX:42008X0730(01)&from=DE
Auszug aus 2010 vorgenommenen Änderungen an der Regelung Nr. 30 der Wirtschaftskommission für Europa der Vereinten Nationen (UN/ECE) — Einheitliche Bedingungen für die Genehmigung der Luftreifen für Kraftfahrzeuge und ihre Anhänger
1.ANWENDUNGSBEREICH
Diese Regelung umfasst neue Luftreifen, die hauptsächlich, aber nicht nur, für Fahrzeuge der Klassen M1, N1, O1 und O2 bestimmt sind. Sie gilt nicht für Reifen, die hauptsächlich bestimmt sind für
a) die Ausrüstung historischer Fahrzeuge (Oldtimer);
b) Wettbewerbe.
Quelle: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:307:0001:0001:DE:PDF
Einlagerung von Reifen
Vor dem Einlagern sollte der Luftdruck der Reifen um ca. 0,5 bar gegenüber der Herstellervorgabe erhöht werden. Auch Reifen die nicht benutzt werden, verlieren langsam an Druck.
Lagern Sie Ihre Reifen kühl, trocken, dunkel und ohne chemische oder mechanische Beeinflussung. Zusätzlich ist es wichtig, das jeglicher Dreck wie Streusalzreste, o.ä. vor der Lagerung vom Reifen entfernt werden, da diese Rückstände schnell zu Alterserscheinungen und Problemen am Reifen führen können.
Kompletträder (Reifen auf Felge montiert) können Sie liegend übereinander stapeln. Alternativ können Sie auch einen Felgenbaum nutzen. Reifen ohne Felge können Sie stehend lagern: Drehen Sie die Reifen alle paar Wochen um einige Grad, damit sich die Reifen nicht verformen.
Außerdem empfiehlt sich eine Messung der Profiltiefe. Der Gesetzgeber schreibt 1,6 mm Restprofil vor. Die Empfehlung des ADAC ist Sommerreifen bei 3 Millimeter und Winterreifen bei 4 Millimeter Restprofiltiefe zu tauschen.
EU-Reifenlabel: EU-Reifen-Kennzeichnungs-Verordnung
Ab dem 1. November 2012 müssen nach EU Verordnung Reifen mit einem Label versehen werden. Reifen, die ausschließlich für die Montage an Fahrzeugen ausgelegt sind, deren Erstzulassung vor dem 1.10.1990 erfolgte, sind von dieser Regelung nicht betroffen und benötigen daher kein EU Reifenlabel. Quelle: http://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=CELEX:42008X0730(01)&from=DE
Auszug aus der VERORDNUNG Nr. 1222/2009 DES EUROPÄISCHEN PARLAMENTS UND DES RATES vom 25. November 2009 über die Kennzeichnung von Reifen in Bezug auf die Kraftstoffeffizienz und andere wesentliche Parameter
(1) Diese Verordnung gilt für Reifen der Klassen C1, C2 und C3.
(2) Diese Verordnung gilt nicht für
a) runderneuerte Reifen;
..
c) Reifen, die ausschließlich für die Montage an Fahrzeugen ausgelegt sind, deren Erstzulassung vor dem 1. Oktober 1990 erfolgte;
Quelle: http://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=uriserv:OJ.L_.2009.342.01.0046.01.DEU
Fabrikatsbindung
Im letzten Jahrhundert wurden in vielen KFZ - Scheinen neben der Reifengröße auch die Reifenmarke und das Profil vorgeschrieben. Die angegebenen Reifen waren durch die Fahrzeughersteller für die Verwendung an den jeweiligen Fahrzeugen geprüft und freigegeben (Homologation). Ab März 2000 wird auf die Eintragung von Reifen - Fabrikats - Bindungen bei Neufahrzeugen verzichtet. Alte Eintragungen sind hinfällig und besitzen nur noch Empfehlungscharakter.
Fahrwerksgeometrie
Zur Fahrwerksgeometrie zählen u. a. Sturz, Spur und Nachlauf. Um nach einer der Umrüstung auf eine andere Reifendimension die optimalen Fahreigenschaften zu erhalten, ist oftmals eine Anpassung der Fahrwerksgeometrie unerlässlich. Wird dies nicht beachtet, kann es zu negativen Auswirkungen auf das Fahrwerk und durchaus auch zu Beeinträchtigung der Sicherheit kommen.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Feinwuchten
Hierbei werden die Restunwuchten, die durch geänderte Radzentrierung, Nabe und Bremsscheibe auftreten direkt am Fahrzeug ausgeglichen.
Felgengröße
Die international gebräuchlichen Größenangaben für Felgen bezeichnen die Radbreite von Felgenhorn zu Felgenhorn sowie den Durchmesser des Rades und die Form des Felgenhorns. Beispiel: Felge Pagode (Bj. 63-67): 5.5 J x 14
5.5 Zoll beschreibt die Radbreite, 14" ist der Durchmesser und J das Felgenhorn.
Eine Hump-Sicherheitsfelge erkennen Sie an der H1 (1 Hump) oder H2 (Doppelhump) - Kennung.
Felgentypen: Tiefbettfelgen, Halbtiefbettfelgen, Steilschulterfelgen, Hochschulterfelge
Felgenschutz
Die Felgenschutzleiste ist ein Gummistreifen, welcher an der Seitenwand etwa in Höhe der Reifenwulst rund um den Reifen verläuft.. Diese Gummilippe steht leicht vom Reifen ab und berührt als erster Radbestandteil den Bordstein. So kann die Felge geschützt werden, wenn der Fahrer rechtzeitig reagiert. Folgende Begriffe werden von den Reifenherstellern verwendet oder sind gebräuchlich:
-P: Reifen mit Felgenschutz
ML: Mit Leiste
MFS: Mit Felgenschutz
MFSL: Mit Felgenschutzleiste
RPB: Rim Protection Bar
FP: Fringe Protector
FR: Felgenrippe
Fülldruck
Der Reifenfülldruck beeinflusst unmittelbar wichtige Faktoren wie Fahrsicherheit, Spritverbrauch und Reifenverschleiß. Unerklärlich, auch unter ökonomischen Gesichtspunkten, ist daher die Tatsache, dass nur jeder 4. Autofahrer auf unseren Straßen mit optimalem Luftdruck unterwegs ist. Bei 10% der Autofahrer liegt der Minderdruck sogar bei mehr als 0,6 bar unter Normal. Aufgrund von Diffusion durch die Seitenwand verliert ein Reifen stets etwas Luft. Obwohl der Anteil verschwindend gering ist, summiert sich der Luftverlust im Laufe der Zeit auf ein gefährliches Maß. Die Folgen: Seitenführungskräfte im Reifen stehen nicht mehr ausreichend zur Verfügung, das Fahrzeug reagiert verzögert auf Lenkbewegungen, Geradeauslauf und Bremsweg verschlechtern sich. Das wirkliche Ausmaß dieser Mängel wird zumeist erst in Gefahrenzonen deutlich, wenn die Gesamtheit der Fahreigenschaften erforderlich ist, um das Fahrzeug sicher zu steuern. Ist ein Reifen mit zu wenig Luft befüllt, vergrößert sich die Aufstandsfläche, wobei die Druckverteilung der Radlast stark auf die äußeren Ränder verteilt und in der Mitte der Fläche verringert wird. Aufgrund der unterschiedlichen Kräfteverteilung entsteht, abhängig von Geschwindigkeit und Gewicht des Fahrzeugs eine mitunter starke Walkbewegung mit Wärmeentwicklungen von über 150°C. Im schlimmsten Fall überhitzt das Material im Schulterbereich, der Unterbau verliert seine Festigkeit, es lösen sich Teile von Lauffläche und Gürtel ab. Über die Sicherheitsmängel hinaus sind die direkten Folgen für den Geldbeutel des Autofahrers nicht außer acht zu lassen. Bereits 0,2 bar Minderdruck verringert die Lebensdauer eines Reifens um etwa 15%, 0,6 bar Minderdruck um immerhin 45%. Darüber hinaus wird durch die höhere Walkarbeit der Rollwiderstand erhöht, was zwangsläufig den Kraftstoffverbrauch steigert. Der Luftdruck sollte immer bei kalten Reifen geprüft werden, da er durch die Erwärmung des Reifens um bis zu 0,5 bar ansteigt. Angaben zum richtigen Luftdruck findet man in der Tankklappe, am Türholm oder auf jeden Fall in der Betriebsanleitung seines Fahrzeugs. Über den "normalen" Luftverlust hinaus gibt es auch Ursachen für einen verstärkten Luftverlust im Reifen. Eingefahrene Gegenstände in der Lauffläche, Verletzung der Seitenwand oder ein defektes Ventil können bei hohen Belastungen zum Totalschaden des Reifens führen. Schmutz, Staub und Feuchtigkeit können die Funktionsweise des Ventils beeinträchtigen, daher ist immer darauf zu achten, dass das Ventilkäppchen fest angeschraubt ist. Seit einigen Jahren werden im Reifenfachhandel auch speziell dichtende Gase als Reifenfüllungen angeboten, die überwiegend im Fernverkehr eingesetzt werden. Diese Gase entweichen nicht durch die Seitenwand und können den einmal eingestellten Fülldruck etwa ein Jahr halten. Beschädigungen am Reifen oder am Ventil können jedoch auch damit nicht ausgeschlossen und vor allem nicht kompensiert werden. Die sicherste und kostengünstigste Methode ist letztendlich die regelmäßige manuelle Überprüfung des Luftdrucks an der Tankstelle. Nicht zu vergessen ist dabei das Ersatzrad, das man gelegentlich kontrollieren sollte. Hierbei sollte der Luftdruck um 0,5 bar höher sein als beim Gebrauchsreifen. Der Wert kann im Bedarfsfall einfach auf den jeweils benötigten Wert abgesenkt werden, nachträgliches Aufpumpen hingegen ist nicht einfach möglich. Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Füllmittel für Reifen
In der Praxis des Reifenfachhandels werden jährlich viele Reifen mit speziellen Einsatzzwecken ausgeschäumt. Dies dient der Absicherung gegen Reifenpannen. Laut BRV lehnen jedoch alle Reifenhersteller die Gewährleistung für derart behandelte Reifen ab.
Ganzjahresreifen
Ganzjahresreifen gehen einen Kompromiss ein, welcher den sehr spezifischen Stärken der Sommer- und Winterreifen unterlegen ist. Ganzjahresreifen haben eine M+S Kennung und können sogar ein Schneeflockensymbol tragen. Allerdings haben auch die besten Ganzjahresreifen im Winter einen längeren Bremsweg als Winterreifen. Auf Grund dessen sind Ganzjahresreifen nur empfehlenswert, wenn Sie wirklich wenig (d.h. weniger als 15.000 km pro Jahr) fahren. Unsere Empfehlung für Deutschland: Winterreifen im Winter, Sommerreifen im Sommer.
Geschwindigkeitsindex / Speedindex (SI)
Der Geschwindigkeitsindex oder "Speed-Index" bezieht sich auf die Höchstgeschwindigkeit des Reifens in Abhängigkeit von der Tragfähigkeit. Der auf dem Reifen befindliche SI kann einer höheren Geschwindigkeit entsprechen, darf aber nicht den im Fahrzeugschein angegebenen Wert unterschreiten.
Alle aufgeführten Höchstgeschwindigkeiten verstehen sich inklusive Toleranz. Der Geschwindigkeitskennbuchstabe auf der Seitenwand gibt nicht nur die zulässige Höchstgeschwindigkeit einer Bereifung an, sondern hat auch maßgeblichen Einfluss auf die maximale Tragfähigkeit.
Geschwindigkeits-Kennbuchstaben
| Kenn- buchstabe |
km/h |
| A1 | 5 |
| A2 | 10 |
| A3 | 15 |
| A4 | 20 |
| A5 | 25 |
| A6 | 30 |
| A7 | 35 |
| A8 | 40 |
| B | 50 |
| C | 60 |
| Kenn- buchstabe |
km/h |
| D | 65 |
| E | 70 |
| F | 80 |
| G | 90 |
| J | 100 |
| K | 110 |
| L | 120 |
| M | 130 |
| N | 140 |
| P | 150 |
| Kenn- buchstabe |
km/h |
| Q | 160 |
| R | 170 |
| S | 180 |
| T | 190 |
| U | 200 |
| H | 210 |
| V | 240 |
| VR | >210 |
| W | 270 |
| ZR | >240 |
| Y | 300 |
Die Höchstgeschwindigkeit eines Pkw ergibt sich aus der Höchstgeschwindigkeit laut Fahrzeugschein (Ziffer 6) plus einer TÜV-Toleranz von 6,5 km/h + 0,01 x Höchstgeschwindigkeit - max.! Faustformel: Fahrzeughöchstgeschwindigkeit + 9 km/h
Auszug aus der Richtlinie 97/24/EG des Europäischen Parlaments und des Rates vom 17. Juni 1997 über bestimmte Bauteile und Merkmale von zweirädrigen oder dreirädrigen Kraftfahrzeugen
2.1.12. Reifen, die für Geschwindigkeiten über 240 km/h geeignet sind, müssen innerhalb der Größenbezeichnung des Reifens vor der Angabe der Bauart (siehe Abschnitt 2.1.3) mit dem jeweils zutreffenden Kennbuchstaben "V" oder "Z" gekennzeichnet werden (siehe Abschnitt 1.31.3).
2.1.13. Reifen, die für Geschwindigkeiten über 240 km/h (bzw. 270 km/h) geeignet sind, müssen in Klammern die für eine Geschwindigkeit von 210 km/h (bzw. 240 km/h) geltende Tragfähigkeitskennzahl und folgenden Bezugskennbuchstaben für die Geschwindigkeitskategorie aufweisen:
- "V" im Falle von Reifen, die innerhalb der Größenbezeichnung den Kennbuchstaben "V" aufweisen;
- "W" im Falle von Reifen, die innerhalb der Größenbezeichnung den Kennbuchstaben "Z" aufweisen.
Quelle: http://eur-lex.europa.eu/legal-content/DE/TXT/?uri=CELEX:31997L0024&qid=1448910100227
Gürtel
Unter der Lauffläche, direkt über der Karkasse, liegen mehrere Cord-ähnliche Lagen aus dünnen Stahldrähten. Diese so genannten Gürtelfäden liegen im spitzen Winkel zur Lauffläche des Reifens. Der Gürtel sorgt für die Stabilität des Reifens und optimiert eine Vielzahl von Eigenschaften, wie die Verringerung des Rollwiderstandes und dadurch die Temperatur im Reifen. Der gesamte Reifen wird fahrstabil, die Lenkpräzision ist hervorragend. Die Stahlcordfäden sind zum Schutz gegen Rost und um eine bessere Verbindung zum Kautschuk zu erreichen, vermessingt. Zum Schutz der Gürtellage wird, je nach Ausführung, eine Messing- oder Kautschuk Beschichtung aufgetragen, die den Stahlcord gegen Rost schützt und die Verbindung zum Gummi unterstützt. Trotzdem kann bei einer Beschädigung des Reifens kann Feuchtigkeit eindringen und der Stahlgürtel kann rosten. Die Folge: Es kann zu gefährlichen Gürtelablösungen kommen. Der Gürtel wurde bereits aus verschiedenen Materialien hergestellt. So verwendete man in den Anfängen der Reifenherstellung Textilcord. In modernen Hochleistungsreifen werden heute sogar Aramidfasern (Kevlar-Kohlestofffasern) verwendet. Letztendlich ist die Verwendung des Stahlgürtels heute jedoch die Regel und wird in der Reifenherstellung praktiziert.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Haltbarkeit von Reifen
Reifen altern aufgrund physikalischer und chemischer Prozesse beispielsweise durch Witterungseinflüsse wie UV-Licht, Feuchtigkeit sowie extrem hohen oder niedrigen Temperaturen. Dadurch verändern sich Elastizität und Haftfähigkeit des Reifens. Das gilt auch für nicht oder wenig benutzte Reifen. Um diesem Prozess entgegenzuwirken, werden dem Material Substanzen zugegeben, die den Alterungsprozess stark verlangsamen. Damit ist gewährleistet, dass auch ein maximal fünf Jahre alter, sachgemäß gelagerter Reifen der Spezifikation eines Neureifens entspricht und in seiner Verwendungstauglichkeit nicht beeinträchtigt ist.
Herstellung
Grundlage der Reifenproduktion ist zunächst die Herstellung der Kautschukmischungen für verschiedene Funktionen am Reifen. In parallelen Verfahren wird der Laufstreifen gespritzt sowie Textilcord, Wulst und Stahlcord gummiert. Aus dem gummierten Stahlcord werden anschließend Endlosstreifen geschnitten, die wiederum später zur Herstellung der Gürtellagen verwendet werden. Der gummierte Textilcord und der gummierte Wulst werden zur Karkasse, also zum Grundgerüst des Reifens zusammengefügt. Doch so einfach, wie es klingt ist es natürlich nicht, zahlreiche Anforderungen und Bestimmungen sind zu beachten, die Arbeitsabläufe sind im Detail um ein Vielfaches komplexer. Trotz automatisierter Abläufe in der Reifenindustrie, ist die Handarbeit immer noch nötig, um einen Reifen zu produzieren, dieser Anteil beträgt durchschnittlich ca. 30 bis 35 Prozent, je nach Produkt und Reifengröße. Je größer ein Reifen ist, umso mehr Handarbeit muss getätigt werden
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Höhenschlag
Man spricht vom so genannten "Höhenschlag", wenn Rad und Reifen in der Drehbewegung von der Seite betrachtet, eine horizontale Abweichung in der Kreisbewegung aufweisen. Je höher die Geschwindigkeit desto mehr verursacht der Höhenschlag Vibrationen oder Erschütterungen, die sich auf das Fahrwerk und die Karosserie übertragen. Manchmal kann Matchen, das Harmonisieren von Reifen und Felge notwendig sein. Dabei wird der Reifen auf der Felge so lange gedreht bis der Höhenschlag ausgeglichen ist.
Homologation
Homologation ist die technische Freigabe eines Reifens für ein Kraftfahrzeug. Hierbei müssen rund 60 Prüfkriterien beachtet werden.
Hump Der Begriff Hump ist englisch und bedeutet übersetzt Buckel oder Höcker. Die Humps sitzen auf beiden Seiten der Felgenschulter. Sie hindern den aufgezogenen Reifen daran ins Felgenbett zu rutschen. Für Reifen mit Notlaufeigenschaften werden oft EH-Felgen ( EH= Extended Hump), also Felgen mit höheren Humps empfohlen, da sie den Reifen im Notfall noch besser auf der Felge halten.Innenseele / Innerliner
Unter Innerliner oder Innenseele versteht man die luftdichte Gummischicht, welche innen auf die Karkasse aufgebracht wird. Sie sorgt für die Abdichtung des Reifens und macht den Einsatz eines Schlauches im Reifen unnötig. Reifen mit der Aufschrift Tubeless (Schlauchlos) dürfen nicht mit einem Schlauch gefahren werden. Erfordert allerdings die humplose Felge einen Schlauch, müssen schlauchlose Reifen mit Schlauch gefahren werden.
Kalander
Der Kalander ist ein Walzensystem, das sowohl Textilfasern als auch Stahlgewebe für die Reifenproduktion dünn mit Kautschuk beschichtet. Die Ummantelung muss gewährleisten, dass die so genannten Cordlagen sich mit den restlichen Bauteilen optimal verbinden. Dies dient zum einen dem reibungslosen Produktionsablauf, gewährleistet aber letztendlich ein hochwertiges Produkt, das auch Extrembedingungen standhält.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Karkasse
Die Karkasse ist ein wesentlicher Bestandteil des Reifens und besteht aus feinen Cordfäden, früher aus Baumwolle, heute hauptsächlich aus Kunstfaser (im wesentlichen Rayon). Die Karkasse verleiht dem Unterbau Festigkeit und beeinflusst die Faktoren Fahreigenschaft und Komfort. Der Reifenunterbau besteht meist aus einer oder mehreren Karkasslagen, die den entscheidenden Festigkeitsträger bilden. An den Enden wird die Karkasse umgeschlagen, um den Wulst aufzunehmen. Vom Verlauf der Cordfäden von Wulst zu Wulst hängt die Reifenbauart ab: Sind die Fäden schräg zur Fahrtrichtung angeordnet, handelt es sich um Diagonalreifen, wenn sie quer angeordnet sind, also im rechten Winkel zur Laufrichtung, spricht man von Radialreifen. PKW-Reifen enthalten immer eine Radialkarkasse, wobei bei Motorrad- und Flugzeugreifen auch eine Mischbauweise zum Einsatz kommt.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Kennzeichnung von Reifen
135 / 80 R 15 72 Q M+S Reifenbreite in Millimeter
135 / 80 R 15 72 Q M+S Flankenhöhe in Prozent der Reifenbreite
135 / 80 R 15 72 Q M+S Radialreifen
135 / 80 R 15 72 Q M+S Felgendurchmesser in Zoll
135 / 80 R 15 72 Q M+S Tragfähigkeitsindex
135 / 80 R 15 72 Q M+S Geschwindigkeitskennbuchstabe
135 / 80 R 15 72 Q M+S Kennzeichnung von Allwetter- und Winterreifen
5.20 - 13 70 M TL M+S Reifenbreite in Zoll ('')
5.20 - 13 70 M TL M+S Diagonalreifen
5.20 - 13 70 M TL M+S Felgendurchmesser in Zoll
5.20 - 13 70 M TL M+S Tragfähigkeitsindex
5.20 - 13 70 M TL M+S Geschwindigkeitskennbuchstabe
5.20 - 13 70 M TL M+S Tubeless (Schlauchloser Reifen)
5.20 - 13 70 M TL M+S Kennzeichnung von Allwetter- und Winterreifen
Kautschuk
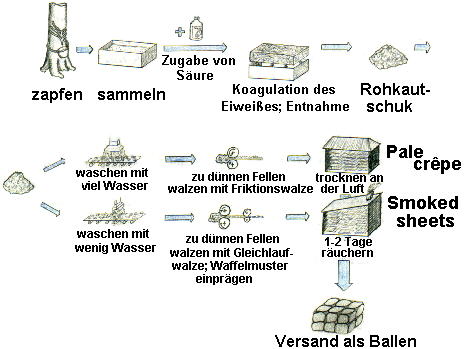 Vom Naturkautschuk zum Gummi:
Vom Naturkautschuk zum Gummi:
Das Ursprungsland des Naturkautschuks ist der brasilianische Urwald auf dem südamerikanischen Kontinent. Dort kommt Kautschuk in sehr hohen Anteilen (ca. 35%) in dem weißlichen Saft eines Baumes namens Hevea brasiliensis vor. Schon die Azteken ritzten die bis zu 60m hohen Pflanzen am Stamm an, um den Saft, auch Latex oder Gummimilch genannt, zu gewinnen. Heutzutage wird die Hevea brasiliensis weltweit im sogenannten Kautschukgürtel (30º nördl.Breite bis 30º südl.Breite) kultiviert, angebaut und regelmäßig geerntet.
Zum Ernten wird dem Baumstamm gräten- oder schraubenartig ein Streifen Rinde entfernt, so dass das Latex in darunter hängende Eimer tropfen kann. Es wird dann in größeren Behältern gesammelt und durch Zugabe von Säuren, meist Essig-, Ameisen- oder Oxalsäure, zur Koagulation gebracht (Ausklumpen des im Latex enthaltenen Eiweiß, in dem das Kautschuk eingelagert ist).
Die so gewonnenen Rohkautschukklumpen werden mit Wasser gewaschen und mit mit anderen Rohkautschuk-Sorten vermischt. Anschließend wird diese Kautschukmasse entweder mit Friktionswalzen oder Gleichlaufwalzen und Waffelmuster zu dünnen Fellen gewalzt. Nach Trocknung (Pale crêpe) oder Räucherung (Smoked sheets) wird der Rohkautschuk als Ballen fertig zum Versand.
Kraftstoffverbrauch
Durch ein angepasstes und vorausschauendes Fahrverhalten kann der Verbrauch von Kraftstoff verringert werden:
- Reifenluftdruck erhöhen (0,1 - 0,2 bar mehr, als vom Hersteller angegeben)
- Im richtigen Moment schalten (fast untertourig fahren)
- Vorausschauend fahren (Gang raus bei roter Ampel)
- Motor aus (beim Warten vor Bahnübergängen)
- Zündkontakte säubern
- Möglichst schnell auf die angestrebte Geschwindigkeit beschleunigen
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Lauffläche von Reifen
Die Lauffläche eines Reifens ist die einzige Verbindung zur Fahrbahn. Sichtbar ist jedoch nur der mit einem Profil versehenen Laufstreifen. Die stabilisierende Schicht darunter bleibt verborgen. Die Lauffläche überträgt alle vom Fahrzeug ausgehende Kräfte.
Die Aufstandsfläche des Reifens bezeichnet den Teil der Lauffläche, welcher den Kontakt zum Untergrund bzw. der Fahrbahn herstellt. Durchschnittliche PKW Reifen verfügen über eine Aufstandsfläche welche in etwa der Größe einer Postkarte entspricht.
Laufleistung
Laufleistung bezeichnet die Lebensdauer eines Reifens bis zur Verschleißgrenze. Viele Faktoren beeinflussen die Laufleistung. Je nach Fahrzeug, der Fahrweise, der Beladung und der Fahrstrecke variiert der Verschleiß. Fahrzeuge mit Frontantrieb verschleißen die Vorderreifen meist deutlich früher als die Hinterreifen. Der Reifenfachhandel empfiehlt das Wechseln des Sommerreifens bei einem Restprofil von 3 mm, ein Wechsel der Winterreifen bei 4 mm Restprofiltiefe. Die gesetzlich vorgeschriebene Restprofiltiefe beträgt 1,6 Millimeter
Laufrichtung / Laufrichtungsbindung
Laufrichtungsbindung: Bestimmte Reifenprofile und Karkassen werden so konstruiert, dass sie in der vorgeschriebenen Laufrichtung abrollen müssen. Diese Reifen verfügen über eine entsprechende Kennzeichnung. Meist in Form eines Pfeils, der montiert außen zu sehen sein muss.
Es gibt Reifen, die keine Laufrichtungsbindung. Diese tragen jedoch eine Markierung „inside“ und „outside“. Bei diesen Reifen muss „outside“ vom Fahrzeug weg zeigen.
Loadindex, Lastindex oder Tragfähigkeitsindex
Der Lastindex besteht aus einer zwei- bis dreistelligen Zahl am Ende der Größenbezeichnung eines Reifens. Hierbei handelt es sich um eine Kennzahl, die die Tragfähigkeit des Reifens wiedergibt. Diese muss immer gleich oder höher sein als der im Fahrzeugschein angegebene LI.
Beispiel: Michelin 205/70 R 15 VR 90W TL XWX
90 entspricht 600 Kg. Bei zwei Reifen darf die zulässige Achslast Ihres Fahrzeugs 1.200 Kg nicht überschreiten.
|
Index |
max kg |
Index |
max kg |
Index |
max kg |
Index |
max kg |
Index |
max kg |
Index |
max kg |
Index |
max kg |
||||||
|
0 |
45 |
30 |
106 |
60 |
250 |
90 |
600 |
120 |
1400 |
150 |
3350 |
180 |
8000 |
||||||
|
1 |
46,2 |
31 |
109 |
61 |
257 |
91 |
615 |
121 |
1450 |
151 |
3450 |
181 |
8250 |
||||||
|
2 |
47,5 |
32 |
112 |
62 |
265 |
92 |
630 |
122 |
1500 |
152 |
3550 |
182 |
8500 |
||||||
|
3 |
48,7 |
33 |
115 |
63 |
272 |
93 |
650 |
123 |
1550 |
153 |
3650 |
183 |
8750 |
||||||
|
4 |
50 |
34 |
118 |
64 |
280 |
94 |
670 |
124 |
1600 |
154 |
3750 |
184 |
9000 |
||||||
|
5 |
51,5 |
35 |
121 |
65 |
290 |
95 |
690 |
125 |
1650 |
155 |
3875 |
185 |
9250 |
||||||
|
6 |
53 |
36 |
125 |
66 |
300 |
96 |
710 |
126 |
1700 |
156 |
4000 |
186 |
9500 |
||||||
|
7 |
54,5 |
37 |
128 |
67 |
307 |
97 |
730 |
127 |
1750 |
157 |
4125 |
187 |
9750 |
||||||
|
8 |
56 |
38 |
132 |
68 |
315 |
98 |
750 |
128 |
1800 |
158 |
4250 |
188 |
10000 |
||||||
|
9 |
58 |
39 |
136 |
69 |
325 |
99 |
775 |
129 |
1850 |
159 |
4375 |
189 |
10300 |
||||||
|
10 |
60 |
40 |
140 |
70 |
335 |
100 |
800 |
130 |
1900 |
160 |
4500 |
190 |
10600 |
||||||
|
11 |
61,5 |
41 |
145 |
71 |
345 |
101 |
825 |
131 |
1950 |
161 |
4625 |
191 |
10900 |
||||||
|
12 |
63 |
42 |
150 |
72 |
355 |
102 |
850 |
132 |
2000 |
162 |
4750 |
192 |
11200 |
||||||
|
13 |
65 |
43 |
155 |
73 |
365 |
103 |
875 |
133 |
2060 |
163 |
4875 |
193 |
11500 |
||||||
|
14 |
67 |
44 |
160 |
74 |
375 |
104 |
900 |
134 |
2120 |
164 |
5000 |
194 |
11800 |
||||||
|
15 |
69 |
45 |
165 |
75 |
387 |
105 |
925 |
135 |
2180 |
165 |
5150 |
195 |
12150 |
||||||
|
16 |
71 |
46 |
170 |
76 |
400 |
106 |
950 |
136 |
2240 |
166 |
5300 |
196 |
12500 |
||||||
|
17 |
73 |
47 |
175 |
77 |
412 |
107 |
975 |
137 |
2300 |
167 |
5450 |
197 |
12850 |
||||||
|
18 |
75 |
48 |
180 |
78 |
425 |
108 |
1000 |
138 |
2360 |
168 |
5600 |
198 |
13200 |
||||||
|
19 |
77,5 |
49 |
185 |
79 |
437 |
109 |
1030 |
139 |
2430 |
169 |
5800 |
199 |
13600 |
||||||
|
20 |
80 |
50 |
190 |
80 |
450 |
110 |
1060 |
140 |
2500 |
170 |
6000 |
200 |
14000 |
||||||
|
21 |
82,5 |
51 |
195 |
81 |
462 |
111 |
1090 |
141 |
2575 |
171 |
6150 |
201 |
14500 |
||||||
|
22 |
85 |
52 |
200 |
82 |
475 |
112 |
1120 |
142 |
2650 |
172 |
6300 |
202 |
15000 |
||||||
|
23 |
87,5 |
53 |
206 |
83 |
487 |
113 |
1150 |
143 |
2725 |
173 |
6500 |
203 |
15500 |
||||||
|
24 |
90 |
54 |
212 |
84 |
500 |
114 |
1180 |
144 |
2800 |
174 |
6700 |
204 |
16000 |
||||||
|
25 |
92,5 |
55 |
218 |
85 |
515 |
115 |
1215 |
145 |
2900 |
175 |
6900 |
205 |
16500 |
||||||
|
26 |
95 |
56 |
224 |
86 |
530 |
116 |
1250 |
146 |
3000 |
176 |
7100 |
206 |
17000 |
||||||
|
27 |
97,5 |
57 |
230 |
87 |
545 |
117 |
1285 |
147 |
3075 |
177 |
7300 |
207 |
17500 |
||||||
|
28 |
100 |
58 |
236 |
88 |
560 |
118 |
1320 |
148 |
3150 |
178 |
7500 |
208 |
18000 |
||||||
|
29 |
103 |
59 |
243 |
89 |
580 |
119 |
1360 |
149 |
3250 |
179 |
7750 |
209 |
18500 |
Luftdruck
Zu niedriger Luftdruck führt zu einer starken Erwärmung des Reifens. Reifenschäden, schlechte Fahrstabilität, höherer Reifenverschleiß, höherer Kraftstoffverbrauch oder ein längerer Bremsweg können die Folge sein. Daher ist es wichtig regelmäßig, z.B. bei jedem Tankstopp den Luftdruck überprüfen. Da Luft schleichend austreten kann, ist eine Änderung im Fahrverhalten nicht unbedingt spürbar. Ein korrekter Reifenfülldruck sorgt für ein Abrollen mit der ganzen Lauffläche auf der Fahrbahn. Das Profil fährt sich gleichmäßig ab und sie erzielen die maximale Kilometerleistung, haben die größte Haftfläche, den minimalen Bremsweg und optimale Kurvenstabilität bei bestem Fahrkomfort.
Bitte gehen Sie bei einer Messung sowie der Einstellung des Luftdruckes Ihrer Reifen wie folgt vor:
- Messen Sie den Luftdruck immer am kalten Reifen
- Korrigieren Sie den Luftdruck am kalten Reifen und nicht am Betriebswarmen Reifen
- die Luftdrücke sollten achsweise gleich sein, können allerdings zwischen der Vorder- und Hinterachse differieren.
- die Ventilkappen sollten immer fest aufgeschraubt sein. Sie verhindern das Eindringen von Staub und Schmutz und schützen das Ventil vor schmutzbedingter Undichtigkeit. Ersetzen Sie eine fehlende Ventilkappe sofort
Luftverlust
Reifen verlieren immer Luft. Luftmoleküle können selbst bei einem luftdichten Reifen langsam nach außen gelangen. Dadurch nimmt der Luftdruck stetig ab. Luftverlust lässt das Fahrzeug instabil werden, das Fahrverhalten verschlechtert sich deutlich. Besonders kritisch ist der Luftverlust an der Hinterachse, diese dient der Spurführung des Fahrzeuges.
M/C - Kennzeichnung
Mit M/C werden Motorradreifen gekennzeichnet. Ihren Oldtimer - Motorradreifen finden Sie in unserem Online-Shop.
M+S
M+S Reifen sind Pkw-Reifen für den Einsatz bei Matsch und Schnee (Englisch: Mud + Snow). Diese Kennzeichnung wird bei Winter- und Ganzjahresreifen verwendet.
M/T
M/T ist die Bezeichnung für spezielle Gelände- oder Offroad Reifen. (Englisch: Mud Terrain)
Meist werden diese für 4x4 Fahrzeuge verwendet.
Matchen
Matchen ist ein spezielles Reifenmontage-Verfahren. Hierbei wird die Lage des Reifens auf der Felge so optimiert, dass ein optimaler Rundlauf bei minimalstem Höhen- sowie Seitenschlag erzielt wird.
Mischbereifung
Mischbereifung sollte immer vermieden werden. Die gleichzeitige Verwendung verschiedener Reifentypen auf Vorder- und Hinterachse können das Fahrverhalten negativ beeinflussen. Pro Achse sollten ausschließlich die gleichen Reifen verwendet werden.
Millimeterfelgen / Millimeterreifen
TRX ist ein System von Radialreifen, das 1975 von Michelin eingeführt wurde. Es wurde entwickelt, um die möglichen Vorteile der erstmals 1948 eingeführten Radialreifen konsequent nutzen zu können; was durch ein System aus Reifen und speziell dafür entwickelten Felgen ermöglicht wurde. Erstmals gelingt es, die Spannung gleichmäßig auf die ganze Karkasse des Reifens zu verteilen – daher auch die Namensgebung. „TR“ steht für „tension répartie“, also „verteilte Spannung“.
Hierfür war eine Abkehr von der bisher verwendeten Tiefbettfelge erforderlich, deren Konstruktion noch aus der Zeit der Diagonalreifen stammt. Die neu entwickelten Felgen weisen als Hauptmerkmal einen niedrigeren Felgenflansch auf. Durch die spezielle Form von Felge und Reifen entstand eine flachere Krümmung der Reifenwand, ohne die S-förmige Krümmung bisheriger Reifenkonstruktionen. Hierdurch wurde auch die sinnvoll nutzbare Reifenflexibilität erhöht. Durch die niedrigere und nach außen gerichtete Kontur des Felgenhorns hat der Reifen unter Luftdruck einen geringeren Halt auf der Felge als bei konventionellen Felgenformen. Um ein Abrutschen des Reifens nach außen zu verhindern, wurden die TRX-Reifen mit einem umlaufenden Wulst auf der Innenseite des Felgensitzes versehen, durch den ein Abgleiten nach außen verhindert wurde.
Vorteile im Fahrverhalten, gegenüber konventionellen Radialreifen, waren unter anderem:
- Erhöhte Straßenhaftung, durch die gleichmäßigere Druckverteilung auf der Lauffläche.
- Erhöhter Komfort, durch die effizienter genutzte Reifenflexibilität.
Da auf der TRX-Felge nur Reifen mit Sicherheitswulst sicher sitzen, musste verhindert werden, dass konventionelle Reifen auf eine TRX-Felge montiert werden. Aus diesem Grund wurde das metrische Maß verwendet.
Die TRX Reifen sowie weitere Michelin Millimeter - Reifen aus der 400-Serie als auch Reifen von Avon, Pirelli und Vredestein finden Sie in unserem Online-Reifenshop.
Quelle www.wikipedia.de
Negativanteil oder Negativprofil
Als Negativanteil werden die Vertiefungen im Reifenprofil bezeichnet. Die Profilrillen dienen vor allem dazu, das Wasser aus dem Profil abzuleiten. Somit beeinflusst das Negativprofil die Aquaplaning-Eigenschaften eines Reifens maßgeblich. Das Gegenteil dazu bildet das Profil. Dieses wird Positivanteil genannt.
Niederquerschnittreifen
Im Pkw-Bereich wird bei Niederquerschnittreifen auch von "Breitreifen" gesprochen. Gemeint sind damit Reifen mit Höhe/Breite-Verhältnissen ab 50% 0,50 (z.B. 215/50 ZR 17). Die Vorteile solcher Reifen zahlen sich insbesondere für Fahrzeuge höherer Geschwindigkeitsklassen durch höhere Fahrstabilität, bessere Lenkpräzision und die Möglichkeit zum Einbau größerer, wirksamerer Bremsanlagen aus. Breitreifen verfügen meistens über ein besonders ansprechendes Profil-Design, denn die Reifen sollen vor allem sportlich und attraktiv wirken.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Offroad-Reifen
Offroad-Reifen sind speziell für Geländefahrzeuge entwickelte Reifen mit entsprechenden Profilen. Sie sind je nach Ausführung und Verwendung optimal für die Bedingungen von Fahrten abseits der Straße ausgelegt und weniger für den Straßenbetrieb geeignet oder verfügen über weniger gute Offroad – Eigenschaften, dafür jedoch gute Leistungen auf der Straße. Viele Offroad-Reifen haben eine M+S-Kennung.
Positivanteil oder Positivprofil
Als Positivanteil werden die Erhebungen vom Reifenprofil bezeichnet. Die Profilrillen, auch Negativanteil dienen vor allem dazu, das Wasser aus dem Profil abzuleiten. Somit beeinflusst das Negativprofil die Aquaplaningeigenschaften, der Positivanteil das Haftungsverhalten des Reifens.
PR -Kennung (Ply Rating)
Ply Rating (PR) ist eine alte Bezeichnung und beschreibt die Tragfähigkeit eines Reifens.
Die PR-Zahl beschreibt die Festigkeit des verwendeten Karkassenmaterials im Vergleich zu Baumwollcord. Häufig wird hiermit auch die Anzahl der Lagen bezeichnet.
Beispiele: 2, 4, 6, 8, 10 oder mehr PR.
PSI (Reifenluftdruck)
PSI ist ein altes Maß für Druck, das heute v. a. im amerikanischen Sprachraum noch Verwendung findet. PSI ist die Abkürzung für Pound-force per square inch. (Kraft mit der ein Pfund auf eine Fläche von 1x1 Zoll drückt). Gebräuchlicher ist die Angabe des Luftdrucks in BAR. 1 Bar entspricht 14.5038 PSI. 1 PSI entspricht 0.068947 BAR.
Querschnittsverhältnis
Das Querschnittverhältnis beschreibt das Verhältnis von Reifenhöhe zu Reifenbreite in %. Beispiel: Der Reifen in der Dimension 185/80 R 14 hat eine Breite von 185 mm, die Höhe beträgt 80 % der Reifenbreite. Somit beträgt die Reifenflanke ca. 74 mm.
Radialreifen
Die Anordnung der Cordfäden im Reifen bestimmt seine Aufbauart. Bei Diagonalreifen sind die Cordfäden schräg zur Fahrtrichtung angeordnet. Bei Radialreifen sind diese hingegen quer zur Laufrichtung angeordnet. Moderne PKW-Reifen verfügen beinahe immer über eine Radialkarkasse. Bei Motorradreifen kommt auch eine Mischbauweise zum Einsatz, der Diagonal-Gürtelreifen. Offroadmotorräder werden meist mit Diagonalreifen ausgestattet. Die Größenbezeichnung wäre z. B. 100/90-21 57H. Ihren Oldtimer - Radialreifen finden Sie in online in unserem Reifen-Shop.
Ein Bericht aus der Zeit zu den Unterschieden von Diagonalreifen und den "neuen" Radialreifen - vom 14.11.1969 "Gürtelreifen‚Wunderreifen?: Mehr „Spitze“: http://www.zeit.de/1969/46/mehr-spitze/komplettansicht.
Reifenaufstandsfläche
Die Reifenaufstandsfläche bezeichnet den Teil des Reifens, welcher den Kontakt zur Fahrbahn herstellt. Normale PKW Reifen verfügen in der Regel über eine Aufstandsfläche, die in etwa der Größe einer Postkarte entspricht.
Reifengröße(n) im Fahrzeugschein
In Ihrem Fahrzeugschein finden Sie die eingetragenen Reifendimensionen .Alter Fahrzeugschein: Ziffern 20 – 23 / Neuer Fahrzeugschein: Ziffern15.1 und 15.2, bei Fahrzeugen mit 3 Achsen auch 15.3.
Überprüfen Sie bitte vor einer Bestellung, ob die zur Zeit montierte Reifengröße mit der im Fahrzeugschein Eingetragenen übereinstimmt: Bei Fahrzeugen mit neuem Fahrzeugschein kann es zu Abweichungen kommen, da dort nur eine Reifengröße eingetragen ist. Die tatsächlich montierte Reifengröße kann jedoch von der Eintragung abweichen. Wenden Sie sich ggf. an Ihren Fahrzeughersteller, um die möglichen Rad- / Reifenkombinationen zu ermitteln. Oder an DEKRA / TÜV.
Reifenschäden
Reifenschäden können verschiedene Ursachen haben:
- Zu hoher oder niedriger Luftdruck
- Falsch ausgewuchtete Räder
- Verstellte Achsgeometrie (Spur, Sturz, usw.)
- Fremdkörper wie Schrauben, Nägel, Scherben usw.
- Montagefehler
- Schnelles Überfahren von Schlaglöchern, Bordsteinkanten, Steinen oder anderen Hindernissen
- Parken auf einer Bordsteinkante
- Zu hohe Geschwindigkeit
- Überladung des Fahrzeugs
- Kontakt mit Chemikalien, Öl, Kraftstoff
Reifenschulter
Als Reifenschulter wird der Übergang der Lauffläche zur Reifenflanke bezeichnet.
Reserverad
Moderne PKW's sind häufig mit einem kleinen, schmalen Notrad ausgestattet. Dieses sollten Sie nur für den Weg zur nächsten Werkstatt benutzen. Beziehen Sie das Notrad bei der Luftdruckkontrolle, auch wenn es nur alle 120.000 – 150.000 Kilometer zu einer Reifenpanne kommt. Auch nicht benutzte Reifen verlieren Luft. Bei einigen Oldtimern gehörte der außen angebrachte Reservereifen zum optischen Erscheinungsbild. Mehr hierzu finden Sie in einem Artikel auf zwischengas.com.
https://www.zwischengas.com/de/blog/2012/10/25/Es-war-einmal-das-Reserverad.html
Runderneuerte Reifen
Bei diesen Reifen wird auf der Seitenwand ein R oder der Schriftzug Retread aufgebracht. Retread ist englisch und heißt übersetzt runderneuert.
Rollwiderstand
Der Rollwiderstand eines Reifens hat maßgeblichen Einfluss auf den Kraftstoffverbrauch Ihres Fahrzeuges. Je geringer der Rollwiderstand desto weniger Haftreibung, da weniger Kräfte übertragen werden. Dadurch haben die Reifen aber auch weniger Grip. Für die Hersteller ergibt sich hieraus ein Zielkonflikt. Ein Reifen ist immer ein bestmöglicher Kompromiss aus Haftung, Rollwiderstand und Traktion.
Eine Erhöhung des Reifendrucks kann zu einem niedrigeren Rollwiderstand führen. Sie sollten nur bis zu ca. 0,5 Bar über dem empfohlenen Druck erhöhen. Das Fahrzeug könnte auf der Straße hoppeln, der Fahrkomfort leidet. Sie haben weniger Grip und es kann zu einem ungleichmäßigen Reifenverschleiß kommen.
Run Flat Reifen
Verlieren normale Reifen Luft beginnen Sie zu walken. Durch die enorme Hitzeentwicklung wird der Reifen meist in kürzester Zeit nahezu vollständig zerstört. Moderne Reifen mit Notlaufeigenschaften sollen dies verhindern.
Je nach Hersteller werden maximale Geschwindigkeiten und Distanzen angegeben. Die Notlaufeigenschaft wird durch einen verstärkten Reifenaufbau erreicht. Vor allem die Wulst, die Seitenwand sowie der Gürtel sind verstärkt und die verwendeten Gummimischungen sind sehr hitzebeständig. So ein verstärkter Reifen bleibt auch bei Druckluftverlust sehr stabil und springt nicht von der Felge.
Nachteile:
- Schlechtere Federungseigenschaften des Reifens selbst sowie durch die höhere ungefederte Masse
- Schwierigere Montage
- Vergleichsweise teuer in der Anschaffung.
- Wir vulkanisieren keine Weißwandringe auf Run-Flat-Reifen, da diese nur einmalig montiert werden sollten.
Schlauchlose Reifen (Tubeless)
Schlauchlose Reifen verfügen innen über eine abdichtende Gummischicht, welche Innenseele oder auch Innerliner genannt wird. Sie haben die Eigenschaft sich selbst auf der Felge abdichten zu können.
Die Kennzeichnung schlauchloser Reifen am Reifen lautet „Tubeless“ (Englisch für Schlauchlos). Schlauchlose Reifen bieten einige Vorteile und werden daher heute in der Regel verwendet. Die Montage ist leichter, das Gewicht ist geringer und Schlauch und Mantel reiben nicht aneinander.
Schlupf
Schlupf entsteht beim Durchdrehen oder Blockieren eines Rades. Dieser bezeichnet die Abweichung zwischen Radumfang und zurückgelegter Strecke pro Radumdrehung. Bei einem Burnout auf der Stelle beträgt der Schlupf 100%. Ebenso bei einem voll blockierenden Rad.
Schneeflocken Kennzeichnung
Hinter dem Schneeflockensymbol steht eine vereinheitlichte Prüfung mit definierten Kriterien. Hier wird der Reifen mit einem standardisierten Reifen verglichen. Schafft der Reifen bessere Werte als der Vergleichsreifen, erhält er das Schneeflockensymbol. Diese Prüfung wurde nötig, nachdem in den USA fast ausschließlich Reifen mit M+S- Kennung angeboten wurden und die Verbraucher nicht mehr unterscheiden konnten, ob ein Reifen Wintereigenschaften aufwies, oder nicht. Seit etwa fünf Jahren setzt sich das Schneeflockensymbol auch in Europa immer mehr durch und steht mittlerweile als eine Art Gütesiegel für Winterreifen. Ein mit einem Schneeflockensymbol ausgezeichneter Reifen befindet sich in der Regel im oberen Drittel der Leistungsfähigkeit von Winterreifen, wie Zeitschriftentests immer wieder beweisen.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Schrägschulter - Felge
Dieser Felgentyp wird hauptsächlich für Kraftomnibusse oder Lastkraftwagen verwendet.
Der Aufbau einer solchen Felge erleichtert das Aufziehen großer Reifen.
Seitenschlag
Wenn ein Rad beim Ablauf vertikal zur Drehrichtung Abweichungen zur Planebene aufweist, nennt sich dies Seitenschlag. Ein Seitenschlag lässt sich im Idealfall durch Matchen minimieren.
Seitenwand
Die Seitenwand eines Reifens wird Flanke genannt. Je nach Ausführung des Reifens ist sie für mehr Fahrkomfort (meist höhere Flanke) oder stabileres Fahrverhalten (eher niedrigere Flanke) ausgelegt. Auf der Seitenwand finden Sie die wichtigsten Informationen über den Reifen: Reifenart, Herkunft, Produktionsdatum, Reifendimension, Tragfähigkeitsindex, Geschwindigkeitskennbuchstabe und einiges mehr.
Sieben Grad (7°)
Unter der Sieben-Grad-Grenze ist der Temperaturbereich gemeint, für den Winterreifen optimiert sind. Auch moderne Mischungstechnologien können den Spagat zwischen den Anforderungen für den Sommer- und Winterbetrieb nicht meistern. Winterreifen verfügen meist über einen höheren Anteile an Naturkautschuk, denn dieser bleibt im Gegensatz zu dem üblicherweise in Sommerreifen verwendeten künstlichen Kautschukarten selbst bei niedrigen Temperaturen noch flexibel und kann damit besseren Kontakt zur Fahrbahn herstellen.
Slicks
Als Slicks werden profillose Rennreifen bezeichnet (Englisch für glatt). Die Lauffläche eines Slicks ist unprofiliert und vollständig glatt. Rennreifen verwenden verschiedenste Gummimischungen, teilweise sogar an einem Reifen in verschiedenen Bereichen. Slicks haben in der Regel mit Ausnahme der Michelin-TB-Reifen keine Straßenzulassung.
Moderne Sportreifen für Motorräder verfügen zum Teil über verschiedene Laufflächenzonen mit unterschiedlichen Mischungen für optimalen Grip in tiefer Schräglage in Kombination mit hoher Laufleistung. Die Haftungswerte eines Slicks können nur auf trockener Fahrbahn erreicht werden. Ohne Profil wird bei Nässe kein Wasser abgeleitet. Daher werden im Rennsport Intermediates für feuchte und Regenreifen bei nasser Straße verwendet.
Sommerreifen
Sommerreifen sind ganz speziell für den Einsatz bei hohen Temperaturen ausgelegt. Das Profildesign sowie die Gummimischung sind speziell auf diese Anforderungen hin entwickelt worden. Allerdings haben diese Reifen bei Temperaturen unter der 7-Grad-Grenze deutliche Nachteile gegenüber Winterreifen.
Statische Unwucht
Eine statische Unwucht der Rad/Reifenkombination zeigt sich im Rundlauf des Rades. Bei statischer Unwucht springt das Rad förmlich auf der Straße. Ein Rad, welches sich auf einer waagerechten Achse frei drehen kann, muss in jeder beliebigen Position stehen bleiben können. Da die schwerste Stelle des Rades immer nach unten zeigt lässt sich mittels Gegengewicht an der gegenüberliegenden Seite des Rades die Unwucht ausgleichen.
Tread-Wear-Indikator
Bei Kraftfahrzeugen (PKWs, LKWs, Motorräder u. ä.) versteht man darunter die Umwandlung der Antriebskraft in Vortrieb.
Wort
Tread-Wear-Indikator (TWI) ist die Bezeichnung für in der Lauffläche integrierte Abriebindikatoren. Diese bilden schmale durchgehende, 1,6 mm dicke Stege. Der Tread Wear Indicator (TWI) wird im Profil sichtbar, sowie die Mindestprofiltiefe erreicht ist. Die Lage der Indikatoren ist ganz oben auf der Seitenwand gekennzeichnet.
Quelle: Bundesverband Reifenhandel und Vulkaniseur-Handwerk e.V. (BRV)
Übersteuern
Übersteuern ist ein seitliches Ausbrechen des Fahrzeughecks. Ursachen hierfür kann u.a. ein abruptes Gaswegnehmen in einer Kurve sein. Dabei werden die Hinterräder entlastet, erzeugen dadurch weniger Haftreibung und das Fahrzeugheck bricht tangential aus der Fahrspur heraus. Heckgetriebene Fahrzeuge lassen sich häufig durch gezieltes Gasgeben in Kurven zum Übersteuern bewegen. Dies wird kontrolliert ausgeführte Drift genannt. Das Gegenteil zu einem Fahrzeug, welches übersteuert ist ein Fahrzeug, das untersteuert.
Untersteuern
Ein typisches Fahrverhalten bei frontgetriebenen Autos. Untersteuern beschreibt, wenn ein Fahrzeug in Kurven über die Vorderachse tangential zum Kurvenrand schiebt. Das Gegenteil ist Übersteuern. Vereinfacht dargestellt bedeutet Untersteuern zu wenig und Übersteuern zu viel Kurvenradius.
Unwucht
Unwuchten sind Massenungleichgewichte an der Rad / Reifenkombination. Unwuchten werden in der Fachwerkstatt bei der Montage der Reifen auf der Felge auf einer Wuchtmaschine bestimmt und mittels Ausgleichsgewichten neutralisiert.
Ventil
Es gibt unterschiedliche Arten von Ventilen. Gummiventile, die von innen in das vorgesehene Felgenloch eingezogen werden und sich selbst abdichten, sowie Schraubventile, welche zur Abdichtung einen Dichtring verwenden.
Viele Ventile sind gerade ausgeführt, einige weisen jedoch unterschiedliche Winkel auf. In der Regel sind zu 90°.
Ventile erlauben das einfache Prüfen und Füllen des Luftdruckes bei Motorrädern, welche oftmals über sehr große Bremsscheiben verfügen. Für Hochgeschwindigkeitsfahrzeuge werden Ventile aus Metall oder sehr kurz ausgeführte Ventile aus Gummi verwendet. Diese Ventile bieten bei sehr hohen Geschwindigkeiten einen Sicherheitsvorteil, da sie kaum einem fliehkraftbedingten Luftverlust unterliegen. Generell sollte die Ventilkappe montiert sein um das Ventil vor Staub und Feuchtigkeit zu schützen.
Ventilkappe
Die Ventilkappe sollte immer auf dem Ventil verschraubt sein. Sie schützt das Ventil vor Staub und Feuchtigkeit und sollte bei Verlust ersetzt werden.
Verstärkte Reifen
Folgende Unterscheidungen von verstärkten Reifen gibt es:
- C (Commercial): Für Transporter und leichte Nutzfahrzeuge
- CP (Camping): Camping-Fahrzeuge
- LT (Light Truck): Vergleichbar zu C-Reifen
- Reinforced (Englisch für verstärkt): Verstärkter Reifen
- XL-Reifen (Extra Load): Vergleichbar zu Reinforced Reifen
Vulkanisation
Das Kautschuk wird durch Zugabe von Säuren aus der Latex-Milch des Hevea Baumes (bzw. den flüssigen Zutaten der Synthesekautschuke) zu einer relativ festen, aber noch verformbaren Masse. Chemisch gesehen haben sich dabei die einzelnen, frei beweglichen Moleküle zu langen Ketten polymerisiert. Diese Kohlenstoffketten haben Längen zwischen 50 und 350 Atome und sind trotzdem noch relativ frei beweglich, da es keine Bindungen zwischen ihnen gibt.
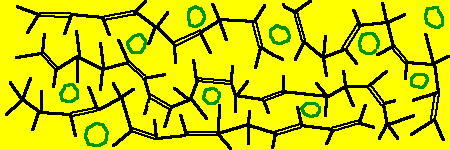 Die Kautschukmasse ist knetbar oder, wie die Chemiker sagen plastisch. Die Ketten können unter äußerer Einwirkung (z.B. Druck) aneinander entlang fließen, sind aber (wegen elektro-statischer Anziehung) ein Festkörper. Gesucht wird für die Reifenvulkanisation aber ein anderer Zustand: Das fertige Gummi soll elastisch sein, also einen Zustand haben, bei der die Form stabil ist.
Die Kautschukmasse ist knetbar oder, wie die Chemiker sagen plastisch. Die Ketten können unter äußerer Einwirkung (z.B. Druck) aneinander entlang fließen, sind aber (wegen elektro-statischer Anziehung) ein Festkörper. Gesucht wird für die Reifenvulkanisation aber ein anderer Zustand: Das fertige Gummi soll elastisch sein, also einen Zustand haben, bei der die Form stabil ist.
Um dies zu erreichen, müssen die Ketten über chemische Brücken untereinander verbunden werden. Dabei hat sich herausgestellt, dass sich Schwefel dafür am besten eignet.
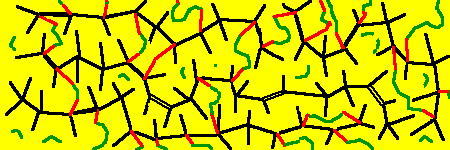 Der Schwefel (in seiner natürlichen festen Form als S8-Molekül) wird als Pulver dem Kautschuk untergemischt. Um nun die Schwefelmolekülringe aufzubrechen und zwischen die Kautschukketten einzulagern, wird viel Energie benötigt. Deshalb findet die Vulkanisation, der Übergang vom plastischen zum elastischen Zustand, effektiv erst bei Temperaturen um 150ºC und Druck statt. Untersuchungen haben dabei ergeben, dass die Schwefelringe aufbrechen (bzw. teilweise auch zerbrechen) und sich zwischen den in den Kohlenstoffketten noch vorhandenen Doppelbindungen anlagern. Dadurch können die Ketten bei äußerer Einwirkung nicht mehr aneinander vorbei fließen.
Der Schwefel (in seiner natürlichen festen Form als S8-Molekül) wird als Pulver dem Kautschuk untergemischt. Um nun die Schwefelmolekülringe aufzubrechen und zwischen die Kautschukketten einzulagern, wird viel Energie benötigt. Deshalb findet die Vulkanisation, der Übergang vom plastischen zum elastischen Zustand, effektiv erst bei Temperaturen um 150ºC und Druck statt. Untersuchungen haben dabei ergeben, dass die Schwefelringe aufbrechen (bzw. teilweise auch zerbrechen) und sich zwischen den in den Kohlenstoffketten noch vorhandenen Doppelbindungen anlagern. Dadurch können die Ketten bei äußerer Einwirkung nicht mehr aneinander vorbei fließen.
Vulkanisation am Beispiel einer Schlauchreparatur
Bei einer Schlauchreparatur tritt folgendes Problem auf: Das schon durchvulkanisierte Gummi ist elastisch und das darin eingelagerte Schwefel nicht mehr fließfähig, das heißt die Schwefelring-Bruchstücke können sich nicht mehr zu den noch bestehenden Doppelbindungen im vulkanisierten Gummi und den Doppelbindungen des neuen Kautschuks hin bewegen.
Wie findet dann eine Verbindung statt?
Genauere Untersuchungen haben ergeben, dass sich tatsächlich (fast) keine Schwefelbrücken zwischen altem und neuem Gummi gebildet haben. Die Haftung dazwischen basiert ausschließlich auf Adhäsionskräften, also Kräften zwischen verschiedenen oder zumindest getrennten Materialien, und nicht auf Kohäsionskräften, die Kräfte innerhalb einer homogenen Masse.
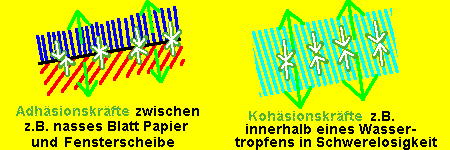 Die Adhäsionskräfte halten nun zwei Oberflächen zusammen, weil diese genau aufeinander passen, ein Ziehen zwischen ihnen also ein Vakuum erzeugen würde, was die Oberflächen zusammen zieht. Vergrößert man nun die Oberflächen (zum Beispiel durch Aufrauhen), sind auch die Adhäsionskräfte größer.
Die Adhäsionskräfte halten nun zwei Oberflächen zusammen, weil diese genau aufeinander passen, ein Ziehen zwischen ihnen also ein Vakuum erzeugen würde, was die Oberflächen zusammen zieht. Vergrößert man nun die Oberflächen (zum Beispiel durch Aufrauhen), sind auch die Adhäsionskräfte größer.
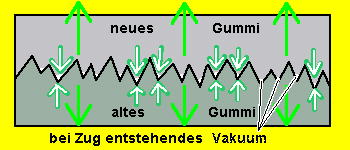 Darauf basiert die Schlauchreparatur. Nach dem Auffinden des Loches wird die Oberfläche angerauht. Um zu verhindern, dass sich nun in die Rillen der Rauhnarbe Luft einschließt, wenn man die Stelle mit neuem Rohkautschuk belegt, wird die aufgerauhte Stelle mit Gummi-Lösung (in Benzol aufgelöstes Rohgummi bzw. Reifenkautschuk) eingestrichen. Dieses Einstreichen verschließt die tiefen Rillen und sorgt zusätzlich dafür, daß das neue Kautschuk bis zum Vulkanisieren auf dem alten Gummi klebt.
Darauf basiert die Schlauchreparatur. Nach dem Auffinden des Loches wird die Oberfläche angerauht. Um zu verhindern, dass sich nun in die Rillen der Rauhnarbe Luft einschließt, wenn man die Stelle mit neuem Rohkautschuk belegt, wird die aufgerauhte Stelle mit Gummi-Lösung (in Benzol aufgelöstes Rohgummi bzw. Reifenkautschuk) eingestrichen. Dieses Einstreichen verschließt die tiefen Rillen und sorgt zusätzlich dafür, daß das neue Kautschuk bis zum Vulkanisieren auf dem alten Gummi klebt.
![]() Dann wird die Stelle in eine passende Heizform geklemmt und unter Druck auf 130º C bis 170º C erhitzt. Die Heizzeit hängt von der Dicke des zu vulkanisierenden Rohgummis ab (etwa 2 bis 5 Minuten pro Millimeter). Beim Vulkanisieren brechen nun die Schwefelringe im Rohgummi und in der getrockneten Gummi-Lösung auf und verbinden deren Kohenstoffketten. Die so entstandene Oberfläche des neuen Gummis paßt genau auf die Rauhnarbe des alten Gummis.
Dann wird die Stelle in eine passende Heizform geklemmt und unter Druck auf 130º C bis 170º C erhitzt. Die Heizzeit hängt von der Dicke des zu vulkanisierenden Rohgummis ab (etwa 2 bis 5 Minuten pro Millimeter). Beim Vulkanisieren brechen nun die Schwefelringe im Rohgummi und in der getrockneten Gummi-Lösung auf und verbinden deren Kohenstoffketten. Die so entstandene Oberfläche des neuen Gummis paßt genau auf die Rauhnarbe des alten Gummis.
![]() Diese Verbindung läßt sich nicht durch Zug- oder Reibungskräfte trennen.
Diese Verbindung läßt sich nicht durch Zug- oder Reibungskräfte trennen.
Der Schlauch ist repariert.
Walken
Reifen aus Gummi werden bei jeder Umdrehung im Bereich der Auflagefläche (Latsch) in ihrem Querschnitt verformt, der Reifen wird regelrecht geknetet. Dreht sich das Rad weiter geht das Gummi in seine ursprüngliche Position zurück. Dieser Vorgang, der den Reifen mechanisch verformt, lässt Reibungswärme entstehen. Die bei dem Vorgang geleistete Arbeit nennt man Walkarbeit.
Weißwandreifen
Unsere nachträglich aufvulkanisierten Weißwandringe machen aus fast jedem Reifen einen Weißwandreifen.
Weißwandreifen für Autos verfügen in der Regel über eine weiße Reifenflanke. Bei Motorradreifen werden zwei weiße Reifenflanken benötigt. Diese Reifen wurden hauptsächlich bei Oldtimer-Fahrzeugen verwendet und werden heute nicht mehr oder nur noch in geringen Stückzahlen von einigen Herstellern produziert. Ursprünglich waren alle Reifen weiß, der Grundstoff Naturkautschuk ist selbst weiß. Erst durch die Verbesserung der Gummimischungen hat sich die Farbe durch die Zugabe von Ruß zu schwarz geändert. Ihren Weißwandreifen finden Sie online in unserem Shop für Oldtimer- und Weißwandreifen.
Winterreifen
Winterreifen verfügen über eine spezielle Gummimischung, welche bei Kälte, Nässe und Schnee eine deutlich bessere Haftung haben als Sommerreifen. Das Profil eines Winterreifens härtet selbst bei niedrigsten Temperaturen nicht aus und kann sich optimal mit der Fahrbahn verzahnen. Als Faustformel für Verwendung von Winterreifen in Deutschland gilt von O bis O, also von Oktober bis Ostern. Der Fachhandel empfiehlt eine Profiltiefe von 4mm. Ihren Oldtimer-Winterreifen finden Sie im Online-Reifen-Shop.
Winterreifenpflicht
Seit dem 04.12.2010 gibt es in Deutschland die situative Winterreifenpflicht für alle im Straßenverkehr befindlichen Kraftfahrzeuge gemäß der Straßenverkehrsordnung.. Die Reifen dieser Fahrzeuge müssen bei Witterungslagen wie Glatteis, schneeglatter Straße, Schneematsch oder Glätte durch Reif das Symbol „M+S“ tragen.
Die zweiundfünfzigste Verordnung zur Änderung straßenverkehrsrechtlicher Vorschriften vom 10. März ist am 31. Mai im Bundesgesetzblatt veröffentlicht worden und somit am 01. Juni 2017 in Kraft getreten. Die Verordnung enthält primär Ergänzungen, Konkretisierungen beziehungsweise Erweiterungen der im Jahr 2010 eingeführten situativen Winterreifenpflicht. Darauf weist der BRV hin.
Alle ab dem 01. Januar 2018 produzierten Reifen müssen mit dem „3 Peak Mountain Snow Flake“ (3PMSF) Piktogramm, also dem Schneeflockensymbol, gekennzeichnet sein, damit diese als Winterreifen gelten. Für die bis zum 31. Dezember 2017 produzierten und nur mit M+S gekennzeichneten Winterreifen gilt eine Übergangsfrist bis zum 30. September 2024. Für die ordnungsgemäße Bereifung des Fahrzeuges mit Winterreifen ist neben dem Fahrzeugführer auch der Fahrzeughalter verantwortlich. Durch die neue gesetzliche Verankerung der Verantwortlichkeit des Fahrzeughalters soll ein Auseinanderfallen der Verantwortlichkeit von Fahrzeugführer und Fahrzeughalter vermieden werden.
Kraftfahrzeuge der Klassen M2 und M3 (Fahrzeuge zur Personenbeförderung mit mehr als acht Sitzplätzen außer dem Fahrersitz) und der Klassen N2 und N3 (Fahrzeuge mit einer zulässigen Gesamtmasse von mehr als 3,5 Tonnen) müssen zukünftig nicht nur auf den permanent angetriebenen Achsen, sondern auch auf den vorderen Lenkachsen mit Winterreifen ausgerüstet werden. Diese Verpflichtung tritt spätestens ab dem 01. Juli 2020 in Kraft. Die Regelung ist jedoch gegebenenfalls ab einem früheren Zeitpunkt anwendbar. Der Gesetzestext der zweiundfünfzigsten Verordnung zur Änderung straßenverkehrsrechtlicher Vorschriften kann im internen Bereich der BRV Homepage heruntergeladen werden unter: Mitglieder-Login à Downloads à Recht à Gesetze und Verordnungen à 52. Verordnung zur Änderung straßenverkehrsrechtlicher Vorschriften (situative Winterreifenpflicht).
http://www.gummibereifung.de/nachrichten/brv-aenderungen-situativen-winterreifenpflicht-gesetzlich-verankert
WL-Kennung
White Letter (Englisch für weiße Buchstaben) – Reifen werden auch heutzutage noch produziert. Amerikanische Hersteller beschriften die Reifenflanke meist mit ihrem Namenszug. Ihren White-Letter-Reifen finden Sie in unserem Reifen-Onlineshop.
Wuchten
Fertigungstoleranzen von Reifen und Felgen verursachen Ungleichgewichte am Rad-Reifen-System. Schon wenige Gramm Unwucht führen im Fahrbetrieb zu Geräuschen, Vibrationen sowie erhöhtem Verschleiß an Radlager, Radaufhängung sowie dem Reifen. Das Auswuchten dient dem individuellen Ausgleich der Massenverteilung an der Rad-Reifen Kombination.
Wulst
Wulst bezeichnet den Teil des Reifens, welcher zwischen dem Hump und dem Horn der Felge für den festen und sicheren Halt des Reifens auf der Felge zuständig ist. Er besteht aus einem Drahtkern, der in Karkassenfäden eingebettet ist und bei der Montage wie ein Ring um die Felge gespannt wird.
Zoll
Zoll ist eine amerikanische Längeneinheit bzw. Längenmaß. Als Größenbezeichnung von Reifen und Felgen hat sich Zoll als Maßeinheit jedoch international durchgesetzt. 1 Zoll = 1 Inch = 25,4 Millimeter

